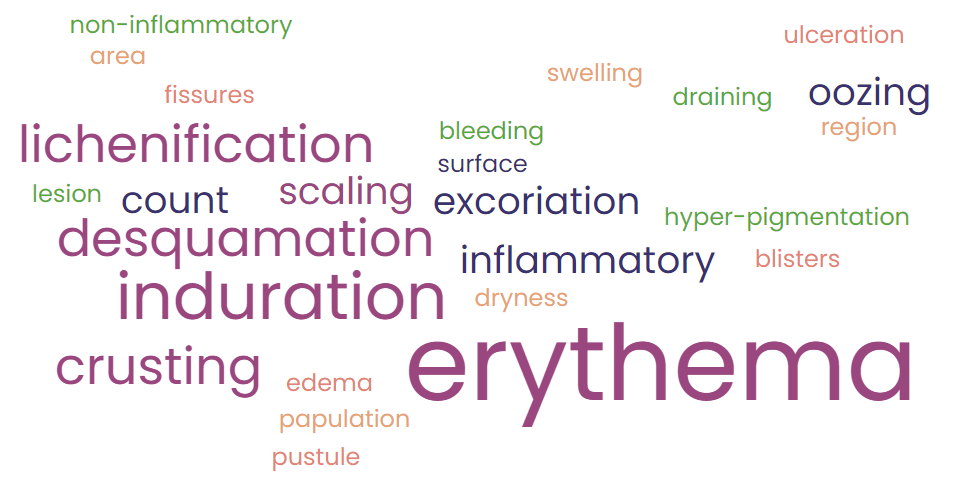
This Word Cloud is the output of descriptors from 12 CLINRO instruments across several well-studied inflammatory skin diseases (ISDs). Erythema is the dominant measurement criteria according to the source scales, from the Greek ‘erythros’, which literally means redness. This demonstrates a definite need to explore how disease activity is defined as well as how it is measured against the variances of skin tonality.
Depending on the degree of pigmentation, redness is significantly less apparent if not obscured by other signs, altogether absent for practical purposes. As a practical matter Erythema may offer diminishing returns as a sign of activity in darker skin tones. To thoroughly address this limitation it will be necessary to recalibrate scale concepts to reflect severity signs as well as quality of life measures.
Thalocan is passionate about improving clarity and precision in clinical trials, believing there is no greater opportunity for improvement in skin disease assessment than in the recognition and evaluation of severity in skin of color. Because of this, Thalocan is striving to support the research community in several other areas:
Efficacy Measure Calibration
Our soon to be released TRI-COA application is a unique ePRO platform designed to capture more complete efficacy data collection and actionable insights to support scale development, validation and adaptation for skin of color.
Scale Adaptation
Scales are often created for a specific project or study, meaning that development and validation projects often simplify or narrow the variables for practical purposes. Once a scale has been reliably demonstrated, it becomes canon in the immuno-derm ecosystem, but may not be analyzed for universal utility in clinical research. Great work has been done in creating a framework for clinical disease measurement and we are building upon that to help Sponsors, CROs and medical societies adapt existing tools to capture assessment more precisely across a broader skin tone spectrum.
Previously we were involved in a scale adaptation project that targeted the validation of a new skin of color SCORAD as prescribed by the International Atopic Dermatitis Society (ISAD) prior to the project stalling due to the pandemic.
This was prior to the recent 2023 FDA diversity guidance where ISAD targeted AD in Sub-Saharan Africa as requiring attention. Not surprisingly, the prevalence of AD in darker pigmented populations is higher than other skin types globally.
Within the next month we’ll also be introducing TAVOS: The Thalocan Assessment Validation Online System that automates the consensus exercise process. Expert committees can be quickly assembled to review images and test scale performance using skin of color images. This will enable the programming of exercises with kappa results delivered in real time with reproducibility exercises created systematically.
Rater Orientation
A variety of socio-economic factors limit access to inflammatory skin disease treatment and many of these diseases disproportionately affect people of color. Dermatologists who have limited experience with patients of color may not have sufficient context to accurately assess severity or even quickly identify a disease. A compounding factor is that Skin of Color disease images that could be used to orient Physicians are much harder to come by. Thalocan has made overcoming these challenges a priority. Our Inflammatory Skin Disease (ISD) image library contains hundreds of examples of disease state images in dark skin tones. In addition, we use A.I. combined with professional creative image enhancement to provide the necessary images to address this need.